 At SAGICO we value the importance of pre-clinical implant testing as one of the most important tools for safety analysis of our implants. We use the industry guidance and vast number of publications on clinical performance and biomechanical testing, as well as international test standards to develop the best test protocol for each individual product. The testing of our spinal implants is a process that we at SAGICO take as serious as our patient’s health. Our implants undergo the rigors of as many tests as possible to eliminate product fatigue and failure, before our surgeons recommend them to their patients.
At SAGICO we value the importance of pre-clinical implant testing as one of the most important tools for safety analysis of our implants. We use the industry guidance and vast number of publications on clinical performance and biomechanical testing, as well as international test standards to develop the best test protocol for each individual product. The testing of our spinal implants is a process that we at SAGICO take as serious as our patient’s health. Our implants undergo the rigors of as many tests as possible to eliminate product fatigue and failure, before our surgeons recommend them to their patients.
SAGICO understands the importance to a potential recipient of one of our implants and the value this has to a patient’s quality of life. Our tests establish important values for the mechanical properties of tensile strength, bend strength, fatigue, durability, torsional capacity and in the end, reliability. By establishing the repeated reliability of this mechanical property data, we have established a standard in which all of our spine implants adhere and the impact on human factors and the biomechanics of the spine.
Our implants have conducted pulsating compressive strength tests on multiple specimens with  varying test frequencies in an attempt to simulate the conditions of load. SAGICO’s minimum standard simulates approximately 2 years in vivo; approximately 7000 cycles per day, or 5 million compressions. Additionally, purely axial tensile tests, compression and flexure tests, and pure or combined torsion load are applied to spinal systems in compliance with standards including ASTM F1717-9, ASTM F2706-8 or ISO 12189-08.
varying test frequencies in an attempt to simulate the conditions of load. SAGICO’s minimum standard simulates approximately 2 years in vivo; approximately 7000 cycles per day, or 5 million compressions. Additionally, purely axial tensile tests, compression and flexure tests, and pure or combined torsion load are applied to spinal systems in compliance with standards including ASTM F1717-9, ASTM F2706-8 or ISO 12189-08.
- ASTM F1717-9 Standard Test Methods for Spinal Implant Constructs in a Vertebrectomy Model
- ASTM F2706-8 Standard Test Methods for Occipital-Cervical and Occipital-Cervical-Thoracic Spinal Implant Constructs in a Vertebrectomy Model
- ISO 12189-8 Implants for surgery – Mechanical testing of implantable spinal devices – Fatigue test method for spinal implant assemblies using an anterior support
These tests are performed under in-vivo (simulated physiological) conditions, with a tempered saline solution using a thermoregulation bath. Practical statistical evaluations of fatigue tests in the finite life range, or high cycle fatigue, and in the transitional range to fatigue strength, or long life fatigue are also conducted. ASTM F 2077 describes a number of different quasistatic and oscillating tests to provide a mechanical comparison of intervertebral disc prosthesis. These include shear, compression and torsion tests, which provide a simplified in-vivo simulation of the loads imposed on such components. These tests completely satisfy the requirements of Standard ASTM F 2077 – Characterization and fatigue of intervertebral body fusion device assemblies. Again, the tests are implemented under in-vivo (simulated physiological) environmental conditions in a temperature conditioning vessel and corrosion-resistant container, suitable for disinfection in autoclaves up to 120° C.
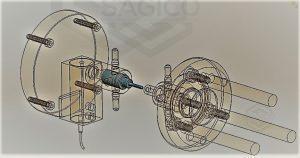 At SAGICO we conduct Torsion Tests on Vertebral Body Implants to ASTM F 2077 and ASTM F1717 Static & Dynamic. We do this because often when comminuted fractures of a vertebral body or tumors in the area of the spinal column it may be necessary to replace a vertebral body with an implant. These vertebral body implants are tested by carrying out quasi-static and oscillating torsion tests in accordance with ASTM F2077 and ASTM F1717. Expulsion testing is also administered, where the expulsion force is determined by positioning the implant between two machined blocks and applying a defined axial preload. A transverse load, which determines the expulsion strength, is then applied to the implant by a testing actuator via a compression die until the implant is expelled. The maximum force determined defines the expulsion force.
At SAGICO we conduct Torsion Tests on Vertebral Body Implants to ASTM F 2077 and ASTM F1717 Static & Dynamic. We do this because often when comminuted fractures of a vertebral body or tumors in the area of the spinal column it may be necessary to replace a vertebral body with an implant. These vertebral body implants are tested by carrying out quasi-static and oscillating torsion tests in accordance with ASTM F2077 and ASTM F1717. Expulsion testing is also administered, where the expulsion force is determined by positioning the implant between two machined blocks and applying a defined axial preload. A transverse load, which determines the expulsion strength, is then applied to the implant by a testing actuator via a compression die until the implant is expelled. The maximum force determined defines the expulsion force.
